Avacta Announces Dose Escalation in the Phase I Clinical Study of AVA6000 Pro-doxorubicin

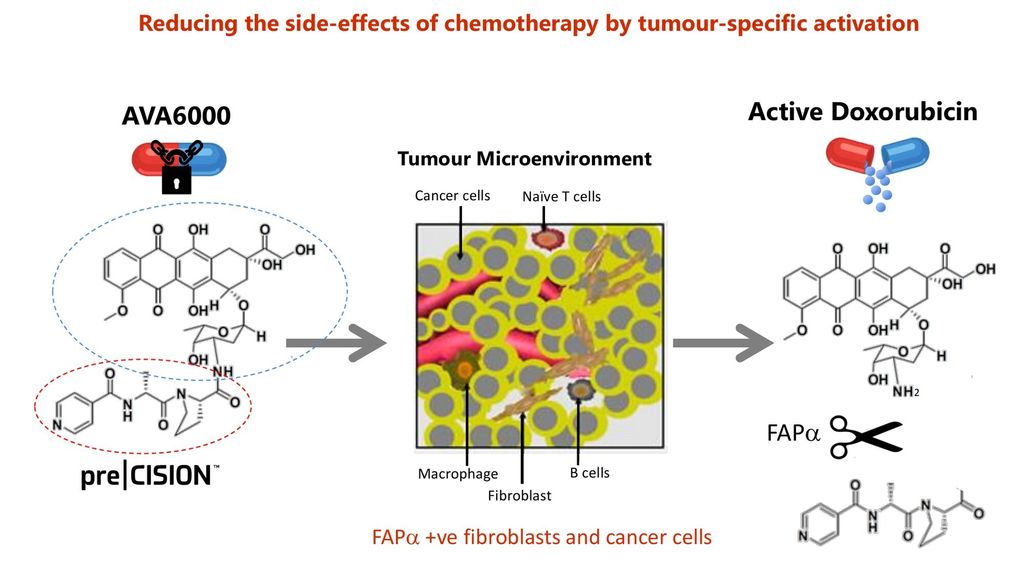
Today’s news that (AVCT is increasing the dosage of its flagship AVA6000 pro-drug in Phase 1 clinical trials by 50% from 80mg/m2 to 120mg/m2 should be viewed as a potential “giant leap for treating cancer” worldwide. Why?
Well in its raw form Doxorubicin is one of the most powerful chemotherapies ever invented. However it’s non-discriminatory. Killing both cancerous & healthy tissues equally - especially around the heart.
That is until possibly now.
After under-going Phase 1 clinical tests (started in Aug'21) of patients with advanced solid tumours. AVCT said its Safety Data Monitoring Committee (SDMC) had concluded AVA6000's safety profile was ‘positive’. Hence the study should continue as planned to the higher dosage level – in order to determine the maximum tolerated dose & recommended Phase II regime.
Meaning AVCT's patented pre|CISION technology already appears to be protecting healthy cells from cross-contamination.
Indeed in comparison, the dosing regime for Doxorubicin is typically limited for safety reasons to a max of 60-75mg/m2 as a monotherapy for only a short period. Whereas AVA6000 seems to handle this fine - & possible much higher.
CEO Alastair Smith adding: "We are delighted with the SDMC's recommendation to move on to the next dose of AVA6000 following its positive review of the safety data.
We are very excited by the potential of AVA6000, &d the pre|CISION platform more broadly, to deliver ground-breaking and affordable cancer treatments that have the potential to significantly improve patients' lives."
Elsewhere further pharmacokinetic (PK) data is expected to be released in due course
Disclaimer & Declaration of Interest
The information, investment views and recommendations in this article are provided for general information purposes only. Nothing in this article should be construed as a solicitation to buy or sell any financial product relating to any companies under discussion or to engage in or refrain from doing so or engaging in any other transaction. Any opinions or comments are made to the best of the knowledge and belief of the writer but no responsibility is accepted for actions based on such opinions or comments. Vox Markets may receive payment from companies mentioned for enhanced profiling or publication presence. The writer may or may not hold investments in the companies under discussion.
